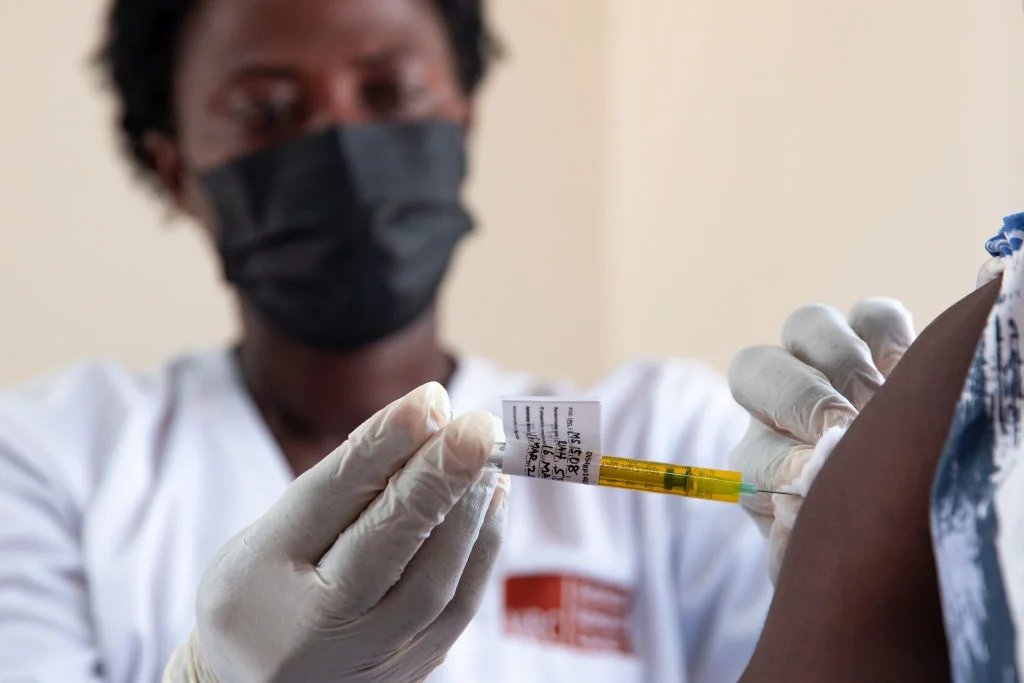
Uganda’s National Drug Authority (NDA) has officially approved the use of lenacapavir, a long‑acting injectable HIV prevention medicine, marking a significant milestone in the country’s efforts to combat HIV/AIDS. In an official post on X (formerly Twitter), the NDA stated, “Uganda’s National Drug Authority has just approved Lenacapavir, a twice‑yearly dose PrEP manufactured by Gilead, a USA based company! This is a game-changer for HIV prevention, especially for those at high risk. This is great step towards ending AIDS by 2030.”